GeneMedicine Oncolytic Adenovirus
Strengths of GM Oncolytic Virus
ㆍThrough 25 years of research, GeneMedicine succeeded in developing the GM Oncolytic viruses that overcome the limitations of existing oncolytic viruses
ㆍ226 SCI-level papers and 157registered patents strongly demonstrate the efficacy and safety of GM Oncolytic viruses
ㆍGM Oncolytic viruses exhibits superior attributes in respect to conventional or competitor oncolytic viruses
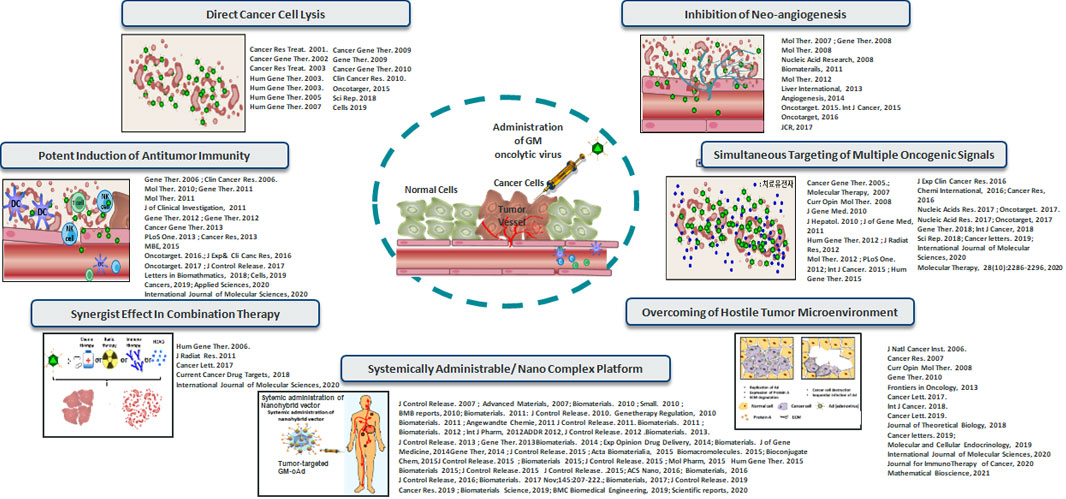
1. Direct Lysis of Cancer Cells
ㆍGM oncolytic viruses replicate effectively and in a highly tumor-specific manner due to proprietary and state-of-the-art genetic modifications
ㆍExcellent tumor selectivity of GM oncolytic viruses minimizes nonspecific replication of the virus in normal tissues, thus achieving superior safety profile over competitor oncolytic viruses
ㆍRelated papers
Cancer Res Treat. 2001; Cancer Gene Ther. 2002; Cancer Res Treat. 2003; Hum Gene Ther. 2003; Hum Gene Ther. 2003; Hum Gene Ther. 2005; Hum Gene Ther. 2007; Cancer Gene Ther. 2009; Gene Ther. 2009; Cancer Gene Ther. 2010; Clin Cancer Res. 2010; Oncotarger, 2015; Sci Rep. 2018; Cells 2019
2. Potent Induction of Anti-tumor Immune Response
ㆍGM oncolytic viruses’ excellent lytic ability in tumor tissues causes ample generation of tumor-associated antigens that leads to robust induction of tumor-specific adaptive immune response. Additionally, GM oncolytic viruses have been armed with extremely potent immune-boosting transgenes that are preferentially expressed at a high level in tumor tissues to inflame the immunosuppressive tumor milieu to induce robust antitumor immune response with minimal off-target side effects
ㆍRelated papers
Mol Ther. 2007; Gene Ther. 2008; Mol Ther. 2008; Nucleic Acid Research, 2008; Biomaterails, 2011; Mol Ther. 2012; Liver International, 2013; Angiogenesis, 2014; Oncotarget. 2015. Int J Cancer, 2015; Oncotarget, 2016; JCR, 2017
3. Synergistic antitumor effect in combination with other cancer therapies
ㆍGM oncolytic viruses in combination with other standard cancer treatment modalities (chemotherapeutics, targeted therapeutics, radiation treatment, and immune checkpoint inhibitors) have been shown to induce synergistic antitumor efficacy across multiple types of tumors
ㆍRelated papers
Gene Ther. 2006; Clin Cancer Res. 2006; Mol Ther. 2010; Gene Ther. 2011; Mol Ther. 2011; J of Clinical Investigation, 2011; Gene Ther. 2012; Gene Ther. 2012; Cancer Gene Ther. 2013; PLoS One. 2013; Cancer Res, 2013; MBE, 2015Oncotarget. 2016; J Exp& Cli Canc Res, 2016; Oncotarget. 2017; J Control Release. 2017; Letters in Biomathmatics, 2018; Cells, 2019; Cancers, 2019; Applied Sciences, 2020; International Journal of Molecular Sciences, 2020
4. Inhibition of Neo-angiogenesis
ㆍGM oncolytic viruses effectively inhibit angiogenesis, which is integral to supplying nutrients and oxygen to rapidly proliferating tumor cells, within tumor microenvironment, ultimately curtailing tumor growth and metastasis
ㆍRelated papers
Cancer Gene Ther. 2005; Molecular Therapy, 2007; Curr Opin Mol Ther. 2008; J Gene Med. 2010; J Hepatol. 2010 ; J of Gene Med, 2011; Hum Gene Ther. 2012; J Radiat Res, 2012; Mol Ther. 2012; PLoS One. 2012; Int J Cancer. 2015; Hum Gene Ther. 2015; J Exp Clin Cancer Res. 2016; Chemi International, 2016; Cancer Res, 2016; Nucleic Acids Res. 2017; Oncotarget. 2017;Nucleic Acid Res. 2017; Oncotarget, 2017; Gene Ther. 2018; Int J Cancer, 2018; Sci Rep. 2018; Cancer letters. 2019; International Journal of Molecular Sciences, 2020; Molecular Therapy, 28(10):2286-2296, 2020
5. Simultaneous Targeting of Multiple Oncogenic Signaling Pathways
ㆍMost conventional oncolytic viruses in clinical development does not express any therapeutic transgene or express only a single therapeutic gene
ㆍGM oncolytic viruses have been engineered to induce robust expression of up to four transgenes at a high level
ㆍThis superior transgene carrying capacity of GM oncolytic viruses in conjunction with proprietary combination of anticancer transgenes guarantees effective and simultaneous targeting of multiple oncogenic signaling pathways to maximize the antitumor efficacy of our virus against heterogenic clinical tumors
ㆍRelated papers
Hum Gene Ther. 2006; J Radiat Res. 2011; Cancer Lett. 2017; Current Cancer Drug Targets, 2018; International Journal of Molecular Sciences, 2020
6. Overcoming the Physical Barriers within Tumors
ㆍGM oncolytic viruses have been designed to effectively overcome the challenges of hostile tumor microenvironment that diminish the therapeutic efficacy of conventional cancer therapeutics and oncolytic viruses. Specifically, GM oncolytic viruses can replicate proficiently under hypoxic tumor microenvironment, whereas conventional oncolytic viruses show diminished replication capacity in the region. Additionally, GM oncolytic viruses have unrivalled ability to degrade aberrantly dense extracellular matrix of tumor tissue to maximize the virus dispersion throughout the entirety of tumor tissues and facilitate penetration and distribution of immune cells and other cancer treatment modalities.
ㆍRelated papers
J Natl Cancer Inst. 2006; Cancer Res. 2007; Curr Opin Mol Ther. 2008; Gene Ther. 2010; Frontiers in Oncology, 2013; Cancer Lett. 2017; Int J Cancer. 2018; Cancer Lett. 2019; Journal of Theoretical Biology, 2018; Cancer letters. 2019; Molecular and Cellular Endocrinology, 2019; International Journal of Molecular Sciences, 2020; Journal for ImmunoTherapy of Cancer, 2020; Mathematical Bioscience, 2021
7. Systemically Administrable Platform Technology
ㆍIntratumoral administration remains the preferred route of administration for conventional oncolytic viruses in clinical setting. Systemic administration of immunogenic oncolytic viruses remain challenging due to inadequate blood retention time, insufficient intratumoral virion accumulation, off-target accumulation in normal organs, and several safety concerns. However, tumor-targeted systemic delivery of oncolytic virus is essential for these viruses to effectively treat disseminated metastases and noninjectable tumors.
ㆍGM’s systemically administrable oncolytic virus platform technology utilizes tumor-targeted nanomaterial to enhance blood retention time, attenuate induction of antiviral immune response, and enable tumor-specific accumulation of systemically administered viruses, thus resulting in potent antitumor effect and excellent safety profile.
ㆍRelated papers
J Control Release. 2007; Advanced Materials, 2007; Biomaterials. 2010; Small. 2010; BMB reports, 2010; Biomaterials. 2011; J Control Release. 2010; Genetherapy Regulation, 2010; Biomaterials. 2011; Angewandte Chemie, 2011; J Control Release. 2011; Biomaterials. 2011; Biomaterials. 2012; Int J Pharm, 2012; ADDR 2012; J Control Release. 2012; Biomaterials. 2013; J Control Release. 2013; Gene Ther. 2013; Biomaterials. 2014; Exp Opinion Drug Delivery, 2014; Biomaterials. J of Gene Medicine, 2014; Gene Ther, 2014; J Control Release. 2015; Acta Biomaterialia, 2015; Biomacromolecules. 2015; Bioconjugate Chem, 2015; J Control Release. 2015; Biomaterials 2015; J Control Release. 2015; Mol Pharm, 2015; Hum Gene Ther. 2015; Biomaterials 2015; J Control Release. 2015; J Control Release. 2015; ACS Nano, 2016; Biomaterials, 2016; J Control Release, 2016; Biomaterials. 2017 Nov;145:207-222.; Biomaterials, 2017; J Control Release. 2019; Cancer Res. 2019; Biomaterials Science, 2019; BMC Biomedical Engineering, 2019; Scientific reports, 2020



 > R&D
> R&D

